Regulatory Intelligence
RAMS Regulatory Intelligence include overviews of each country’s regulatory system to guide your exploration into new markets.
Additionally, you can review medical device definitions, product classification, labeling requirements and a regulatory road-map to gain market approval in countries you’ve targeted.
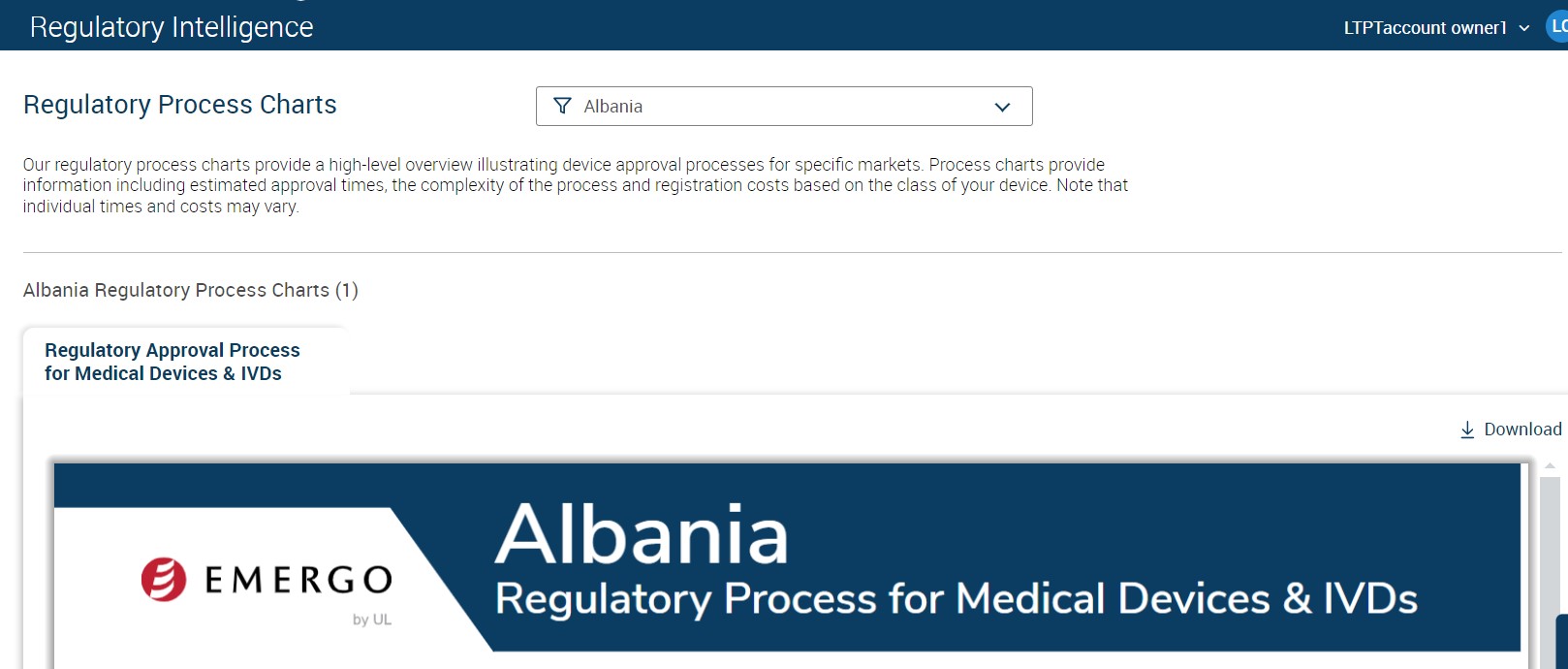
To Access Regulatory Process Charts:
1. Go to Premium Services --> Regulatory Intelligence --> Regulatory Process Charts
2. Select the respective Country
3. Click on "Download" option to download the process chart.
Figure: Regulatory Process Chart
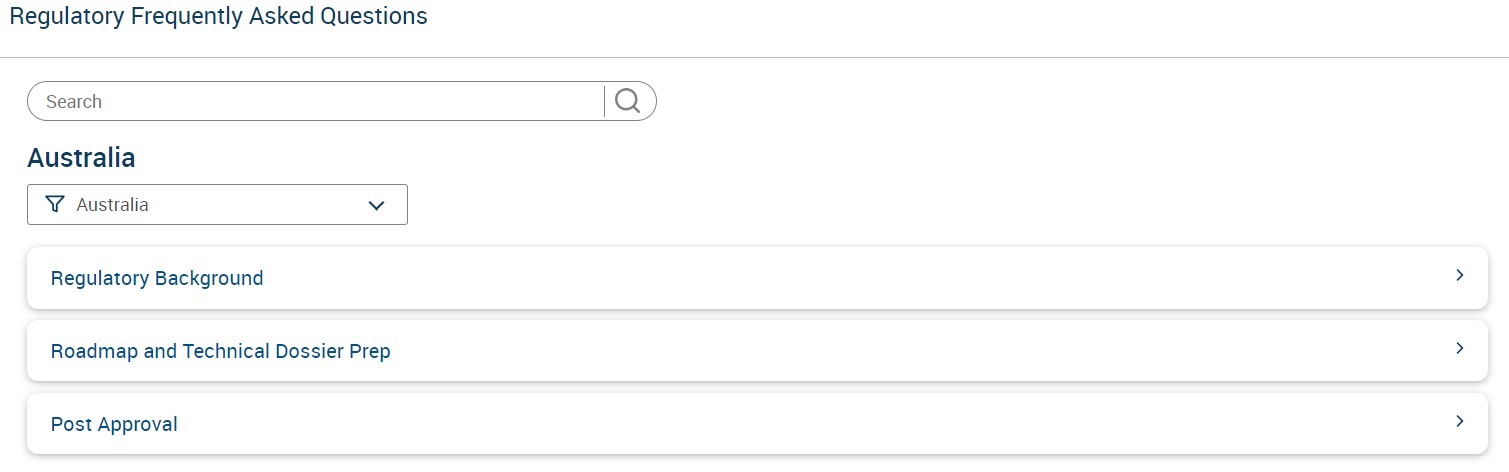
To Access Regulatory FAQs:
1. Go to Premium Services --> Regulatory Intelligence --> Regulatory FAQs
2. Select the country to access Regulatory Background, Roadmap and Post Approval related questions that you might have.